Soap and Detergent
 |
Soaps and detergents contain "surfactants"---compounds with molecules that line up around water to break the "surface tension" that holds it together in drops. They contain a combination of fats (or triglycerides) and alkali that create molecules with two unique chemical ends. One end, the "hydrophilic" end, is attracted to water, and the other, the "hydrophobic" end, is repelled by water but attracted to grease and oil. The hydrophobic ends bond with dirt, and the hydrophilic ends line up around them, encapsulating dirt and grease with a layer of molecules that will allow the dirt to float through the water and ride it down the drain. Surfactants can be anionic (negatively charged) or non-ionic (no charge). Soap, the original surfactant compound, was made with fats such as lard and alkali from wood ashes or lye. Today a wide variety of organic and petroleum-based fatty acids are used, and sodium or potassium hydroxides have replaced wood ash or lye in commercially prepared soaps and detergents.
SOAPS
Soaps are water-soluble sodium or potassium salts of fatty acids. Soaps are made from fats and oils, or their fatty acids, by treating them chemically with a strong alkali.First let's examine the composition of fats, oils and alkalis; then we'll review the soap-making process.
SURFACTANTS IN DETERGENTS
A detergent is an effective cleaning product because it contains one or more surfactants. Because of their chemical makeup, the surfactants used in detergents can be engineered to perform well under a variety of conditions. Such surfactants are less sensitive than soap to the hardness minerals in water and most will not form a film.
Detergent surfactants were developed in response to a shortage of animal and vegetable fats and oils during World War I and World War II. In addition, a substance that was resistant to hard water was needed to make cleaning more effective. At that time, petroleum was found to be a plentiful source for the manufacture of these surfactants. Today, detergent surfactants are made from a variety of petrochemicals (derived from petroleum) and/or oleochemicals (derived from fats and oils).
Anionic Surfactants
The chemical reacts with hydrocarbons derived from petroleum or fats and oils to produce new acids similar to fatty acids.
A second reaction adds an alkali to the new acids to produce one type of anionic surfactant molecule.
Non-ionic Surfactants
non-ionic surfactant molecules are produced by first converting the hydrocarbon to an alcohol and then reacting the fatty alcohol with ethylene oxide.
These non-ionic surfactants can be reacted further with sulphur-containing acids to form another type of anionic surfactant.
Making of Soap
Soap is made two ways. The first, called "saponification," involves cooking fats and adding alkali to the mix at the end to form soap and two byproducts, water and glycerin. The second, called hydrolization, splits the fats and oils into fatty acids and glycerin using steam under pressure. It distills the acids and neutralizes them with alkali. Homemade soap hobbyists make five or six bars of soap using a pound of animal or vegetable oils, two ounces of lye (or other alkali) and about a cup of water. The oils are melted while the akali is added to the water and heated to approximately 110 degrees Fahrenheit. The lye water is added gradually to the cooled oils. The mixture gradually is simmered and re-simmered until a gelatinous substance called "trace" is formed. The trace is poured into molds and must age for several weeks to cure completely.
Making of Detergent
 |
During the past century, the shortage of animal and vegetable fats during World War I and World War II led to the development of detergents, multiple-surfactant, hydrocarbon-based cleaners. Detergents, unlike soap, depend on chemical reactions for their formation. Petroleum-based oils (including "oleo," a compound used to make a replacement for butter) are combined with chemicals such as sulfuric acid, sulfur trioxide or ethylene oxide to form a fatty acid, which is combined with an alkali to form yet another molecule, an anionic surfactant. A second type of process converts a hydrocarbon into a fatty alcohol that then combines with another chemical such as ethylene dioxide to form a nonionic surfactant. Homemade detergents are made by combining a bar of laundry soap such as Fels Naptha or Zote (both of which contain petroleum distillates) with a pound each of washing soda (sodium carbonate) and borax (Sodium borate decahydrate). The combination can be powdered in a blender or the soap can be liquefied with water over heat; then the soda and borax are added to make a liquid.
Process of Soap and Detergent in cleaning
Detergents and soaps are used for cleaning because pure water can't remove oily, organic soiling. Soap cleans by acting as an emulsifier. Basically, soap allows oil and water to mix so that oily grime can be removed during rinsing. Detergents were developed in response to the shortage of the animal and vegetable fats used to make soap during World War I and World War II. Detergents are primarily surfactants, which could be produced easily from petrochemicals. Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely to interact with oil and grease.
Modern detergents contain more than surfactants. Cleaning products may also contain enzymes to degrade protein-based stains, bleaches to de-color stains and add power to cleaning agents, and blue dyes to counter yellowing. Like soaps, detergents have hydrophobic or water-hating molecular chains and hydrophilic or water-loving components. The hydrophobic hydrocarbons are repelled by water, but are attracted to oil and grease. The hydrophilic end of the same molecule means that one end of the molecule will be attracted to water, while the other side is binding to oil. Neither detergents nor soap accomplish anything except binding to the soil until some mechanical energy or agitation is added into the equation. Swishing the soapy water around allows the soap or detergent to pull the grime away from clothes or dishes and into the larger pool of rinse water. Rinsing washes the detergent and soil away. Warm or hot water melts fats and oils so that it is easier for the soap or detergent to dissolve the soil and pull it away into the rinse water. Detergents are similar to soap, but they are less likely to form films (soap scum) and are not as affected by the presence of minerals in water (hard water).
.jpg)

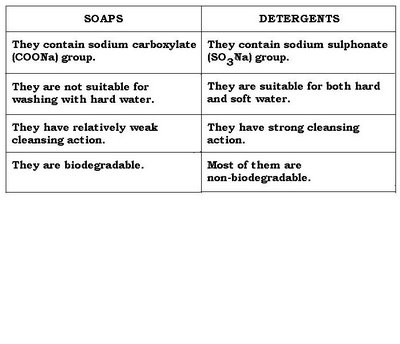
Difference between Soap and Detergent
Soap is a triacylglyceride derviative (fat) while detergents are often produced synthetically. The head-group of soap a molecule is usually a carboxylate anion while common detergents often use phosphate or sulfate head-groups (ie Sodium Dodecyl Sulfate). Soap's effectiveness is greatly affected by the presence of certain minerals in solution such as those typically associated with hard water. Detergents are usually not affected by minerals in solution. The downside to detergents is the fact that most of them are not biodegradable; although some can be chemically modified to allow for degradation by microorganisms usually by the addition of a branched methyl group).
Example of some Soap and Detergent molecule
Example of some Detergent molecule
Detergents and soaps are used for cleaning because pure water can't remove oily, organic soiling. Soap cleans by acting as an emulsifier. Basically, soap allows oil and water to mix so that oily grime can be removed during rinsing. Detergents were developed in response to the shortage of the animal and vegetable fats used to make soap during World War I and World War II. Detergents are primarily surfactants, which could be produced easily from petrochemicals. Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely to interact with oil and grease.
Modern detergents contain more than surfactants. Cleaning products may also contain enzymes to degrade protein-based stains, bleaches to de-color stains and add power to cleaning agents, and blue dyes to counter yellowing. Like soaps, detergents have hydrophobic or water-hating molecular chains and hydrophilic or water-loving components. The hydrophobic hydrocarbons are repelled by water, but are attracted to oil and grease. The hydrophilic end of the same molecule means that one end of the molecule will be attracted to water, while the other side is binding to oil. Neither detergents nor soap accomplish anything except binding to the soil until some mechanical energy or agitation is added into the equation. Swishing the soapy water around allows the soap or detergent to pull the grime away from clothes or dishes and into the larger pool of rinse water. Rinsing washes the detergent and soil away. Warm or hot water melts fats and oils so that it is easier for the soap or detergent to dissolve the soil and pull it away into the rinse water. Detergents are similar to soap, but they are less likely to form films (soap scum) and are not as affected by the presence of minerals in water (hard water).







.jpg)



Its a valuable content shared,would like to know more about it.
ReplyDeletemanufacturers of ethyl hexyl glycerin in mumbai
Useful Information, your blog is sharing unique information....
ReplyDeleteThanks for sharing!!!
buy detergents online pangani
house cleaning products online eastleigh
We also provide analytical services and laboratory services to our customers. copal gum
ReplyDeleteThank you for your post. This is excellent information. It is amazing and wonderful to visit your site.
ReplyDeletebuy meat online pangani
It provides the best step by step guide to reasons to choose the custom soap boxes. I would suggest the people to go through this blog before choosing bathroom tiles. I like how you have researched and presented these exact points so clearly. Check natural bath and body products
ReplyDelete