Organic Chemistry in My Daily Life
Monday, 11 March 2013
Organic Chemistry about Ester
Organic Chemistry about Soap and Detergent
Soap and Detergent
 |
Soaps and detergents contain "surfactants"---compounds with molecules that line up around water to break the "surface tension" that holds it together in drops. They contain a combination of fats (or triglycerides) and alkali that create molecules with two unique chemical ends. One end, the "hydrophilic" end, is attracted to water, and the other, the "hydrophobic" end, is repelled by water but attracted to grease and oil. The hydrophobic ends bond with dirt, and the hydrophilic ends line up around them, encapsulating dirt and grease with a layer of molecules that will allow the dirt to float through the water and ride it down the drain. Surfactants can be anionic (negatively charged) or non-ionic (no charge). Soap, the original surfactant compound, was made with fats such as lard and alkali from wood ashes or lye. Today a wide variety of organic and petroleum-based fatty acids are used, and sodium or potassium hydroxides have replaced wood ash or lye in commercially prepared soaps and detergents.
SOAPS
 |
SURFACTANTS IN DETERGENTS
 |
Anionic Surfactants
Making of Soap
 |
Making of Detergent
 |
Process of Soap and Detergent in cleaning
Detergents and soaps are used for cleaning because pure water can't remove oily, organic soiling. Soap cleans by acting as an emulsifier. Basically, soap allows oil and water to mix so that oily grime can be removed during rinsing. Detergents were developed in response to the shortage of the animal and vegetable fats used to make soap during World War I and World War II. Detergents are primarily surfactants, which could be produced easily from petrochemicals. Surfactants lower the surface tension of water, essentially making it 'wetter' so that it is less likely to stick to itself and more likely to interact with oil and grease.
Modern detergents contain more than surfactants. Cleaning products may also contain enzymes to degrade protein-based stains, bleaches to de-color stains and add power to cleaning agents, and blue dyes to counter yellowing. Like soaps, detergents have hydrophobic or water-hating molecular chains and hydrophilic or water-loving components. The hydrophobic hydrocarbons are repelled by water, but are attracted to oil and grease. The hydrophilic end of the same molecule means that one end of the molecule will be attracted to water, while the other side is binding to oil. Neither detergents nor soap accomplish anything except binding to the soil until some mechanical energy or agitation is added into the equation. Swishing the soapy water around allows the soap or detergent to pull the grime away from clothes or dishes and into the larger pool of rinse water. Rinsing washes the detergent and soil away. Warm or hot water melts fats and oils so that it is easier for the soap or detergent to dissolve the soil and pull it away into the rinse water. Detergents are similar to soap, but they are less likely to form films (soap scum) and are not as affected by the presence of minerals in water (hard water).
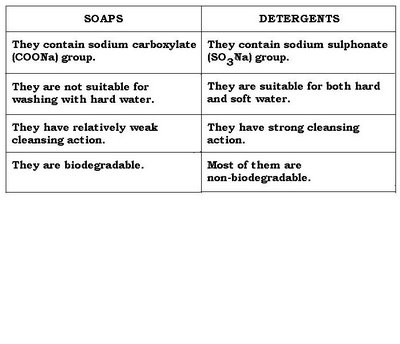
Difference between Soap and Detergent
Soap is a triacylglyceride derviative (fat) while detergents are often produced synthetically. The head-group of soap a molecule is usually a carboxylate anion while common detergents often use phosphate or sulfate head-groups (ie Sodium Dodecyl Sulfate). Soap's effectiveness is greatly affected by the presence of certain minerals in solution such as those typically associated with hard water. Detergents are usually not affected by minerals in solution. The downside to detergents is the fact that most of them are not biodegradable; although some can be chemically modified to allow for degradation by microorganisms usually by the addition of a branched methyl group).

Organic Chemistry about Drugs
Drugs
Where Do Drugs Come From? 61% of the 877 small molecules introduced as drugs worldwide from 1981-
2002 were inspired by “Natural Products” (J. Nat. Prod. 2003, 1022). Hence, the discovery, biological profiling (SAR),
and preparation of natural products is of paramount importance in terms of public health.
Organic chemistry in medical field
Organic chemistry is necessary in the medical fields. All living organisms consist of plenty of organic matter. The organic compounds in the various materials which are vital and important to sustain life. Proteins, carbohydrates and fat are all organic compounds contributed to the structure of the human body. Organic compounds are enzymes and incentive materials that are essential for the occurrence of biological processes. Drugs are also composed mainly of organic compounds. Doctor or pharmacist won’t be effective if he is not sufficiently aware of the structure and function of the organic compounds of organic chemistry and pharmaceuticals and must understand properly the medication description that he offers the patient. It is clear that the organic molecules is necessary to sustain life. Understanding of these compounds is critical in the medical field, not only to understand the basic biological functions, but also to predict the scenarios in the body and which may be due to disruption of organic materials. Completion of a medical organic chemistry can only be achieved understanding of organic chemistry.
Analgesics
Analgesics, also known as "painkillers", are medicines which relieve pain. Most analgesics are safe to use when taken as prescribed or instructed by your doctor or pharmacist, in conjunction with the manufacturer’s instructions on the packaging. Some extra precautions may apply to patients with pre-existing medical conditions such as kidney failure or gastric ulcers.
This page outlines some commonly used over-the-counter analgesics, including what they are used for, possible side effects and risks associated with using them outside the directions on the packet. The painkillers covered are:
- aspirin
- codeine (in combination products)
- ibuprofen
- paracetamol.
Analgesics are available in many forms. These include tablets, capsules, suppositories, soluble powders and liquids. Analgesics are generally swallowed, and their intended purpose is to relieve pain. Some can also be used to reduce fever, help relieve the symptoms of cold and ’flu, reduce inflammation and swelling, control diarrhea, and suppress coughs.
Morphine:
Used as early as 4000 BC, the main ingredient of opium, it was not until 1803 that Morphine was first identified and isolated by the German pharmacist Serturner. He called this alkaloid "Morphia" after Morpheus, the Greek God of Dreams. Morphine is used medicinally to alleviate severe pain. Morphine was
used during the American Civil War as a surgical anesthetic and was sent home with many soldiers for relief of pain. At the end of the war, over 400,000 people had the army disease, morphine addiction. It is obtained from opium poppy pod, used as early as 4000 BC. It has potent analgesic and euphoric properties. Morphine composes 10-15% of dry weight of the poppy. 95% of morphine extracted is converted to codeine.
 | ||
Structure of Codeine
|
Aspirin
 Aspirin and the Willow Tree: The father of modern medicine, Hippocrates, who lived sometime between 460 B.C and 377 B.C. left historical records of pain relief treatments, including the use of powder made from the bark and leaves of the willow tree to heal headaches, pains and fevers. The active ingredient in willow bark, termed salicin, was isolated in 1828, by Buchner, a pharmacy professor of at the University of Munich. By 1829, the French chemist Leroux improved the extraction procedure, obtaining ~30g of salicin from 1.5kg of willow bark. Later, in 1838, the Italian chemist Piria split salicin into a sugar and an aromatic component (salicylaldehyde) and converted the latter, by hydrolysis and oxidation, to a crystalline, colorless acid, that he named “salicylic acid.” However, salicylic acid was tough on stomachs. In 1853, Gerhardt neutralized salicylic acid by buffering it with sodium (sodium salicylate) and acetyl chloride, creating acetylsalicylic acid. Gerhardt had no desire to market his product and abandoned his discovery. In 1899, the German chemist Hoffmann, who worked for Bayer, rediscovered Gerhardt's formula, and gave it to his father who was suffering from arthritis. With good results, Felix Hoffmann convinced Bayer to market the new wonder drug. Aspirin was patented on March 6, 1889.
Aspirin and the Willow Tree: The father of modern medicine, Hippocrates, who lived sometime between 460 B.C and 377 B.C. left historical records of pain relief treatments, including the use of powder made from the bark and leaves of the willow tree to heal headaches, pains and fevers. The active ingredient in willow bark, termed salicin, was isolated in 1828, by Buchner, a pharmacy professor of at the University of Munich. By 1829, the French chemist Leroux improved the extraction procedure, obtaining ~30g of salicin from 1.5kg of willow bark. Later, in 1838, the Italian chemist Piria split salicin into a sugar and an aromatic component (salicylaldehyde) and converted the latter, by hydrolysis and oxidation, to a crystalline, colorless acid, that he named “salicylic acid.” However, salicylic acid was tough on stomachs. In 1853, Gerhardt neutralized salicylic acid by buffering it with sodium (sodium salicylate) and acetyl chloride, creating acetylsalicylic acid. Gerhardt had no desire to market his product and abandoned his discovery. In 1899, the German chemist Hoffmann, who worked for Bayer, rediscovered Gerhardt's formula, and gave it to his father who was suffering from arthritis. With good results, Felix Hoffmann convinced Bayer to market the new wonder drug. Aspirin was patented on March 6, 1889.

Therefore, from all this we can conclude that Organic compounds, lead a very important role in our lives, it is the basic material in our food. Synthetic carbohydrates and protein, fatty acids, vitamins, enzymes and others are only organic compounds, as well as clothing types and components of petroleum and natural gas, organic compounds are important to humans. Scientists were able to manufacture many of the organic compounds that have a key role in our daily lives; such as medicines, pesticides, fertilizers, cosmetics, plastics, and detergents, etc... which has had the greatest impact on human progress.
Organic Chemistry in Modern Life
At first glance, the term "organic chemistry" might sound like something removed from everyday life, but this could not be further from the truth. The reality of the role played by organic chemistry in modern existence is summed up in a famous advertising slogan used by E. I. du Pont de Nemours and Company : "Better Things for Better Living Through Chemistry."
Often rendered simply as "Better Living Through Chemistry," the advertising campaign made its debut in 1938, just as du Pont introduced a revolutionary product of organic chemistry: nylon, the creation of a brilliant young chemist named Wallace Carothers (1896-1937). Nylon, an example of a polymer, started a revolution in plastics that was still unfolding three decades later, in 1967. That was the year of the film The Graduate , which included a famous interchange between the character of Benjamin Braddock (Dustin Hoffman) and an adult named Mr. McGuire (Walter Brooke):
- Mr. McGuire: I just want to say one word to you… just one word.
- Benjamin Braddock: Yes, sir.
- Mr. McGuire: Are you listening?
- Benjamin Braddock: Yes, sir, I am.
- Mr. McGuire: Plastics.
The meaning of this interchange was that plastics were the wave of the future, and that an intelligent young man such as Ben should invest his energies in this promising new field. Instead, Ben puts his attention into other things, quite removed from "plastics," and much of the plot revolves around his revolt against what he perceives as the "plastic" (that is, artificial) character of modern life.
In this way, The Graduate spoke for a whole generation that had become ambivalent concerning "better living through chemistry," a phrase that eventually was perceived as ironic in view of concerns about the environment and the many artificial products that make up modern life. Responding to this ambivalence, du Pont dropped the slogan in the late 1970s; yet the reality is that people truly do enjoy "better living through chemistry"—particularly organic chemistry.
Definition of organic chemistry
Organic chemistry is the study of carbon and the study of the chemistry of life. Since not all carbon reactions are organic, another way to look at organic chemistry would be to consider it the study of molecules containing the carbon-hydrogen (C-H) bond and their reactions.
Significance of organic chemistry
Organic chemistry is important because it is the study of life and all of the chemical reactions related to life. Several careers apply an understanding of organic chemistry, such as doctors, veterinarians, dentists, pharmacologists, chemical engineers, and chemists. Organic chemistry plays a part in the development of common household chemicals, foods, plastics, drugs, fuels... really most of the chemicals part of daily life.
Importance of organic Chemist
An organic chemist is a chemist with a college degree in chemistry. Typically this would be a doctorate or master's degree in organic chemistry, though a bachelor's degree in chemistry may be sufficient for some entry level positions. Organic chemists usually conduct research and development in a laboratory setting. Projects that would use organic chemists would include development of a better painkilling drug, formulating a shampoo that would result in silkier hair, making a stain resistant carpet, or finding a non-toxic insect repellent.

.jpg)



